Principle of Photoexcited Detachment Method
Photoexcited dissociation occurs through three processes: (1) photoexcitation, (2) bond cleavage, and (3) dissociation.
① Photoexcitation
An electron in a σ orbital (bonding orbital) absorbs light of a wavelength corresponding to the energy difference between the σ and σ* orbitals, becoming excited to the σ* orbital (antibonding orbital).
Using vacuum ultraviolet light with high fluence allows efficient excitation of even thin-film samples with short optical paths.
![]()
②Bond Cleavage
When one electron occupies each of the σ and σ* orbitals, the bond energy becomes the sum of the energy levels of both orbitals. When the bond energy is negative, the σ bond undergoes cleavage.
![]() ③Detachment
③Detachment
The cleavage of σ bonds generates various fragments. Some molecules undergo recombination on the surface. If the generated fragments are not bound within the sample, desorption occurs.
Next, we will explain this using the potential curve (Figure 1).
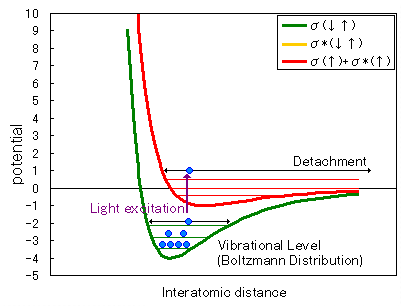
Figure 1: Potential Curve
When two electrons occupy a bonding orbital (Figure 1, green potential curve), they are distributed across each vibrational level according to the Boltzmann distribution.
When vacuum ultraviolet light of a wavelength capable of causing electron transitions strikes this bond, the system transitions to a state where one electron occupies the bonding orbital and one occupies the antibonding orbital (Figure 1, red potential curve).
Molecules with potential energy above zero undergo bond cleavage.
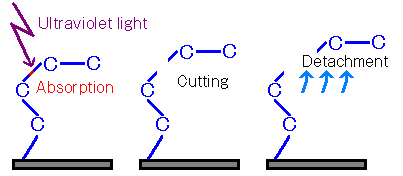
Figure 2: Vacuum Ultraviolet Cutting
On the other hand, when multiple bonds or fragments are bound, the bonds may not be completely cleaved, or even if cleaved, they may not be able to depart.
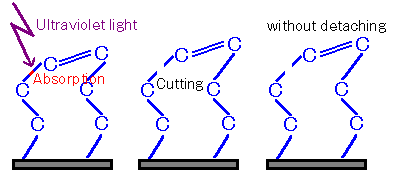
Figure 3: Case ① Where Severance Occurs but Detachment Fails
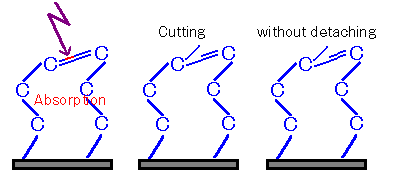
Figure 4: Case ② Where Severance Occurs but Detachment Fails
Contact Us
If you have any questions or concerns about our products,
please feel free to contact us using the inquiry form below.
