Analysis Simulation
1. Factors Affecting Signal Shape
Parameters such as activation energy, frequency factor (vibrational term), number of initial molecules for the desorption component, and heating rate alter the signal shape and peak temperature.
This is evident from the model equations for temperature-programmed desorption shown below.
For the meaning of these equations, please refer to ⇒“Chapter 3: Calculating Desorption Rates in Surface Reactions (pdf/635KB)”.
First-order surface desorption reaction
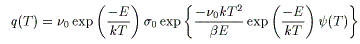
![]()
Surface desorption reactions other than the first-order reaction
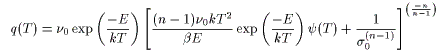
![]()
Diffusion Departure
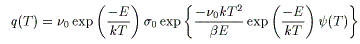
![]()
q(T) is the desorption rate, ν₀ is the frequency factor, D₀ is the vibrational term, σ₀ is the initial number of molecules, C₀ is the initial concentration, n is the reaction order, d is the film thickness, E is the activation energy, k is Boltzmann's constant, β is the heating rate, and T is the absolute temperature. ψ(T) is the series derived by integrating the Arrhenius equation.
The simulation data calculated from these equations is shown below.
Here, ψ(T) = 1 was assumed.
The horizontal axis of each graph represents temperature (K), and the vertical axis represents desorption rate (molecs./sec).
![]()
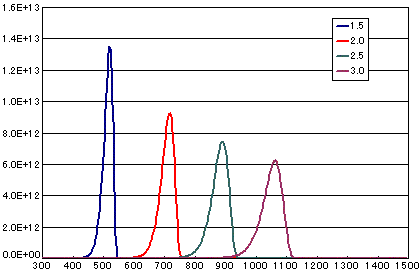
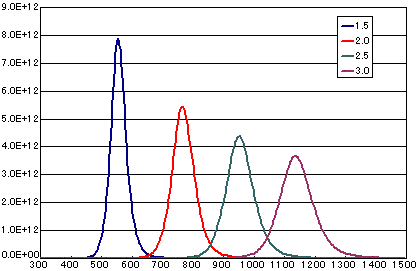
2. Differences due to activation energy
In the case of a first-order dissociation reaction
| A | B | C | D | |
| Activation energy (eV) | 1.5 | 2.0 | 2.5 | 3.0 |
| Frequency factor(sec-1) | 1E13 | 1E13 | 1E13 | 1E13 |
| Number of initial molecules(molecs./cm2) | 1E15 | 1E15 | 1E15 | 1E15 |
| heating rate(K/sec) | 0.5 | 0.5 | 0.5 | 0.5 |
In the case of a secondary elimination reaction
| A | B | C | D | |
| Activation energy (eV) | 1.5 | 2.0 | 2.5 | 3.0 |
| Frequency factor (cm2/molesc.sec) | 1E-3 | 1E-3 | 1E-3 | 1E-3 |
| Number of initial molecules(molecs./cm2) | 1E15 | 1E15 | 1E15 | 1E15 |
| Heating rate(K/sec) | 0.5 | 0.5 | 0.5 | 0.5 |
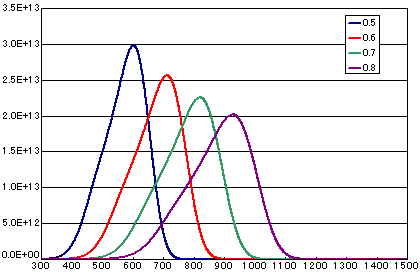
In the case of diffusion-limited processes
| A | B | C | D | |
| Activation energy (eV) | 0.5 | 0.6 | 0.7 | 0.8 |
| Vibration term (cm2/sec) | 1E-1 | 1E-1 | 1E-1 | 1E-1 |
| Number of initial molecules(molecs./cm2) | 1E16 | 1E16 | 1E16 | 1E16 |
| Heating rate(K/sec) | 0.5 | 0.5 | 0.5 | 0.5 |
![]()
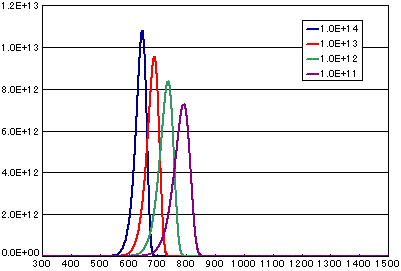
3. Differences due to frequency factors (vibration terms)
In the case of a first-order dissociation reaction
| A | B | C | D | |
| Activation energy (eV) | 2.0 | 2.0 | 2.0 | 2.0 |
| Frequency factor (cm2/sec) | 1E14 | 1E13 | 1E12 | 1E11 |
| Number of initial molecules(molecs./cm2) | 1E15 | 1E15 | 1E15 | 1E15 |
| Heating rate(K/sec) | 0.5 | 0.5 | 0.5 | 0.5 |
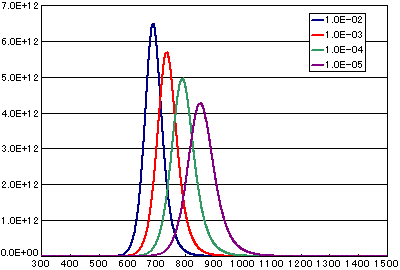
In the case of a secondary elimination reaction
| A | B | C | D | |
| Activation energy (eV) | 2.0 | 2.0 | 2.0 | 2.0 |
| Frequency factor (cm2/molesc.sec) | 1E-2 | 1E-3 | 1E-4 | 1E-5 |
| Number of initial molecules(molecs./cm2) | 1E15 | 1E15 | 1E15 | 1E15 |
| Heating rate(K/sec) | 0.5 | 0.5 | 0.5 | 0.5 |
In the case of diffusion-limited processes
| A | B | C | D | |
| Activation energy (eV) | 0.6 | 0.6 | 0.6 | 0.6 |
| Frequency term (cm2/sec) | 1E-0 | 1E-1 | 1E-2 | 1E-3 |
| Number of initial molecules(molecs./cm2) | 1E16 | 1E16 | 1E16 | 1E16 |
| Heating rate(K/sec) | 0.5 | 0.5 | 0.5 | 0.5 |
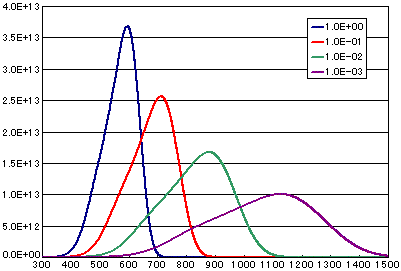
![]()
4. Differences Based on the Number of Initial Molecules
In the case of a first-order dissociation reaction
| A | B | C | D | |
| Activation energy (eV) | 2.0 | 2.0 | 2.0 | 2.0 |
| Frequency factor(sec-1) | 1E13 | 1E13 | 1E13 | 1E13 |
| Number of initial molecules(molecs./cm2) | 2E15 | 1E15 | 5E14 | 2.5E14 |
| Heating rate(K/sec) | 0.5 | 0.5 | 0.5 | 0.5 |
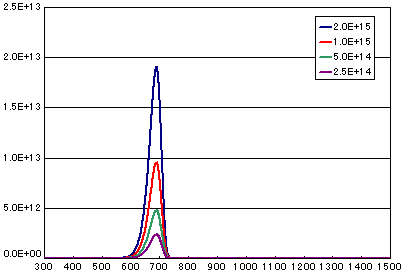
In the case of a secondary elimination reaction
| A | B | C | D | |
| Activation energy (eV) | 2.0 | 2.0 | 2.0 | 2.0 |
| Frequency Factor (cm2/molesc.sec) | 1E-3 | 1E-3 | 1E-3 | 1E-3 |
| Number of initial molecules(molecs./cm2) | 2E15 | 1E15 | 5E14 | 2.5E14 |
| Heating rate(K/sec) | 0.5 | 0.5 | 0.5 | 0.5 |
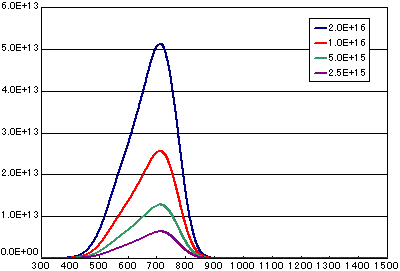
In the case of diffusion-limited processes
| A | B | C | D | |
| Activation energy (eV) | 0.6 | 0.6 | 0.6 | 0.6 |
| Vibration term (cm2/sec) | 1E-1 | 1E-1 | 1E-1 | 1E-1 |
| Number of initial molecules(molecs./cm2) | 2E16 | 1E16 | 5E15 | 2.5E15 |
| Heating rate(K/sec) | 0.5 | 0.5 | 0.5 | 0.5 |
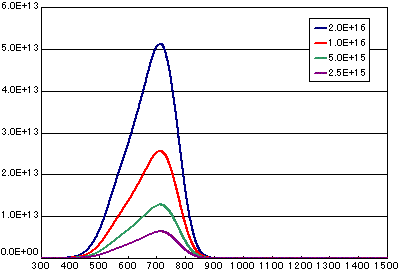
![]()
5. Differences Due to Heating Rate
In the case of a first-order dissociation reaction
| A | B | C | |
| Activation energy (eV) | 2.0 | 2.0 | 2.0 |
| Frequency Factor (sec2) | 1E13 | 1E13 | 1E13 |
| Number of initial molecules(molecs./cm2) | 1E15 | 1E15 | 1E15 |
| Heating rate(K/sec) | 1/6 | 0.5 | 1.0 |
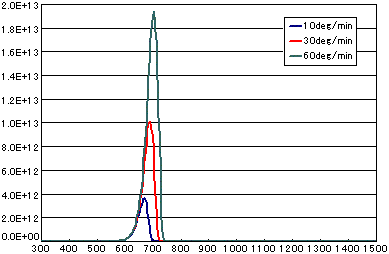
In the case of a secondary elimination reaction
| A | B | C | |
| Activation energy (eV) | 2.0 | 2.0 | 2.0 |
| Frequency Factor (cm2/molesc.sec) | 1E-3 | 1E-3 | 1E-3 |
| Number of initial molecules(molecs./cm2) | 1E15 | 1E15 | 1E15 |
| Heating rate(K/sec) | 1/6 | 0.5 | 1.0 |
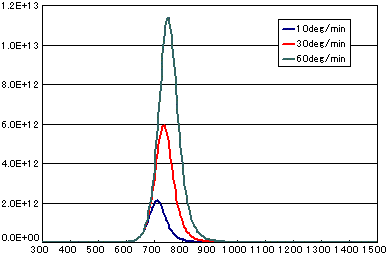
In the case of diffusion-limited processes
| A | B | C | |
| Activation energy (eV) | 0.6 | 0.6 | 0.6 |
| Frequency Factor (cm2/sec) | 1E-1 | 1E-1 | 1E-1 |
| Number of initial molecules(molecs./cm2) | 1E16 | 1E16 | 1E16 |
| Heating rate(K/sec) | 1/6 | 0.5 | 1.0 |
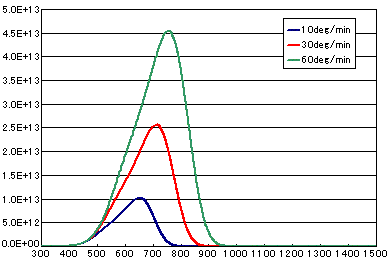
Contact Us
If you have any questions or concerns about our products,
please feel free to contact us using the inquiry form below.
